
All Medical Devices sold in the US, EU and China must carry a Unique Device Identification (UDI) barcode. UDI barcodes are the global standard introduced by Governments to protect patients worldwide. Every UDI code is linked to a public UDI database based on a specific jurisdiction.
A UDI barcode allows every medical device to be tracked across the entire supply chain, from initial manufacture to its use with a patient.
What does UDI stand for?
Unique Device Identification (UDI) is used to identify medical devices. It is regularly found on the packaging and the device or item itself. Depending on its distribution and use, it can be represented as readable text or as a unique numeric/alphanumeric code.
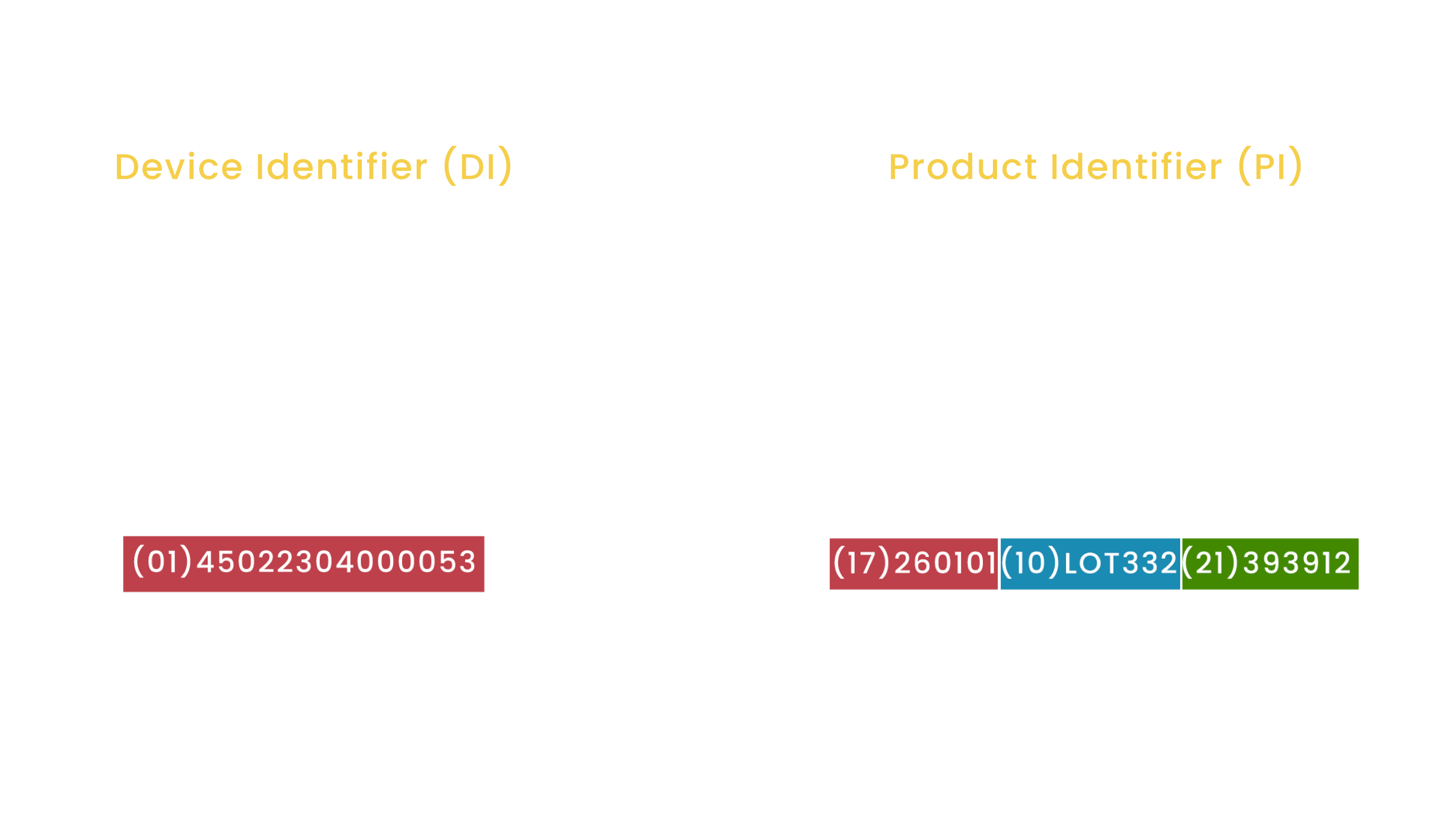
A UDI can be divided into the following parts:
- Device Identifier (DI) - a mandatory prefix that corresponds to the following:
- Manufacturer information
- Model of device
- Production Identifier (PI) - a variable code that represents one or more of the following:
- Device serial number
- Lot number
- Device expiration date
- Date of device manufacture presented in a standard format (YYYY-MM-DD)
- Identification code in case of human cell-based product (HCT/P)
What is the purpose of UDI?
The UDI makes it possible to identify and track medical devices in the supply chain. They are used by the producers and labellers of the products and provide information to healthcare professionals, economic operators, and the wider public.
UDI barcodes are essential when reporting defects or incidents relating to a specific medical device - but they can also be used for inventory monitoring.
A basic UDI or UDI-DI is used to identify and cluster products that share the same design, the same purpose and level of risk. While the UDI-DI does not typically appear on the packaging of a medical device, it must be registered and regulated.
What is a UDI barcode for?
Since 2016, recalls of faulty, expired or defective medical units have increased by over 70% (Statista, 2021). Before introducing the UDI barcode, patients would have to undergo life-threatening surgery to identify if they were at risk from one of these defective devices.
Correct UDI barcode labelling and tracking eliminate this risk by allowing critical data to be obtained upon scanning. This data helps medical staff determine the exact patients implanted with a particular device, track the product journey in the supply chain and ultimately reduce risks related to patient safety.
Do all medical devices need a UDI barcode?
Some countries, like the US, have made it mandatory for all medical devices to have a UDI barcode registered within the global database so that product information can be retrieved as needed. Other countries do not make it mandatory, and the regulations and standards change depending on the region and product. The EU, Australia, China, South Korea and Saudi Arabia have all established jurisdiction-specific requirements for UDI labelling. So, manufacturers and distributors should check the requirements of their jurisdiction.
Are UDI barcodes accepted worldwide?
There are different types of UDI barcodes. The most recommended standards that can issue UDI numbers and generate UDI barcodes are:
GS1: The GS1 UDI barcode standard is recognised in most countries, so it is the most recommended when purchasing a UDI. Product data is supported by the Global Unique Device Identification Database (GUDID), the regulatory basis for UDIs at the FDA in the United States. Once you have applied for your GS1 barcode, you will be assigned a company prefix and a locator that will be used to generate your UDI.
HIBCC: The Health Industry Business Communications Council (HIBCC) is a non-profit organisation responsible for implementing the HIBC UDI barcode. It is currently the regulatory organisation for this standard and is responsible for issuing the Labeller Identification Codes (LICs) that identify individual manufacturers included on each barcode.
ICCBBA: ICCBBA is the organisation that regulates the ISBT128 standard related to products of human origin, whether cellular, tissue or organic. You may consider obtaining the UDI from this organisation if your product is included in this category. Although it does not have the global scope of GS1, it is a standard widely endorsed by the international scientific community.

How can I get a UDI code?
A UDI can be obtained through one of the official issuing agencies operating worldwide. The health authorities accredit each UDI issuing agency in that region.
Organisations looking to obtain a UDI barcode should choose the most appropriate UDI issuing agency for:
- The country they are operating in
- The standards they need to comply with
- The manufacturing properties of the device
In the United States, according to its requirements for issuing UDIs, the FDA accredits the different UDI allocation standards, including GS1, HIBCC and ICCVA.
The European Commission, however, endorses GS1, HIBCC and ICCBBA standards and has also accredited the IFA GmbH in Germany.
In the UK, although the Medical Devices Regulations do not legally require manufacturers to obtain or provide the UDI for medical device registration, many producers have obtained their UDI to market their products internationally.
How to scan UDI barcodes?
Now that you know that UDI can be found in different ways depending on the region or device’s manufacturing properties, you can imagine managing an inventory containing other medical devices is difficult.
At Orca Scan, we have a solution that allows us to scan all these formats, store them, and operate an inventory from anywhere in the world.
The Orca Scan Medical Device Tracking template automatically extracts the Device Identifier, Serial Number, Manufacturer/Production Date, Lot Number and Expiration Date from a UDI barcode into the relevant columns when scanned. In addition, if the medical device is registered with the US FDA, Orca Scan will also populate the manufacturer’s name and device description - reducing human error.
Scan to try the template now 👇

Need help tracking barcodes?
Orca Scan is a GS1 UK-approved barcode tracking app that can be downloaded and installed on any Apple or Android device, including DataLogic, Honeywell and Zebra barcode scanners.
Download the free Orca Scan app and scan any barcode to get started. Any questions? Get in touch, and we’d be happy to help.