Medical Device Tracking: UDI Barcode Scanner
At Orca Scan, we’ve created a UDI barcode scanner solution that allows care providers to track all formats of Unique Device Identification (UDI) barcodes in one single scan. All Medical Devices sold in the US, EU and China must carry a Unique Device Identification (UDI) barcode. UDI barcodes are the global standard, introduced by Governments to protect patients around the world with transparency. A UDI barcode allows every medical device to be tracked across the entire supply chain, from initial manufacture to its use with a patient.
Trusted by over 50,000 organisations in over 165 countries



Minimise Human Error, Maximise Patient Safety
Boost productivity, cut costs, and improve your bottom line.
Full Traceability for Visibility & Compliance
-
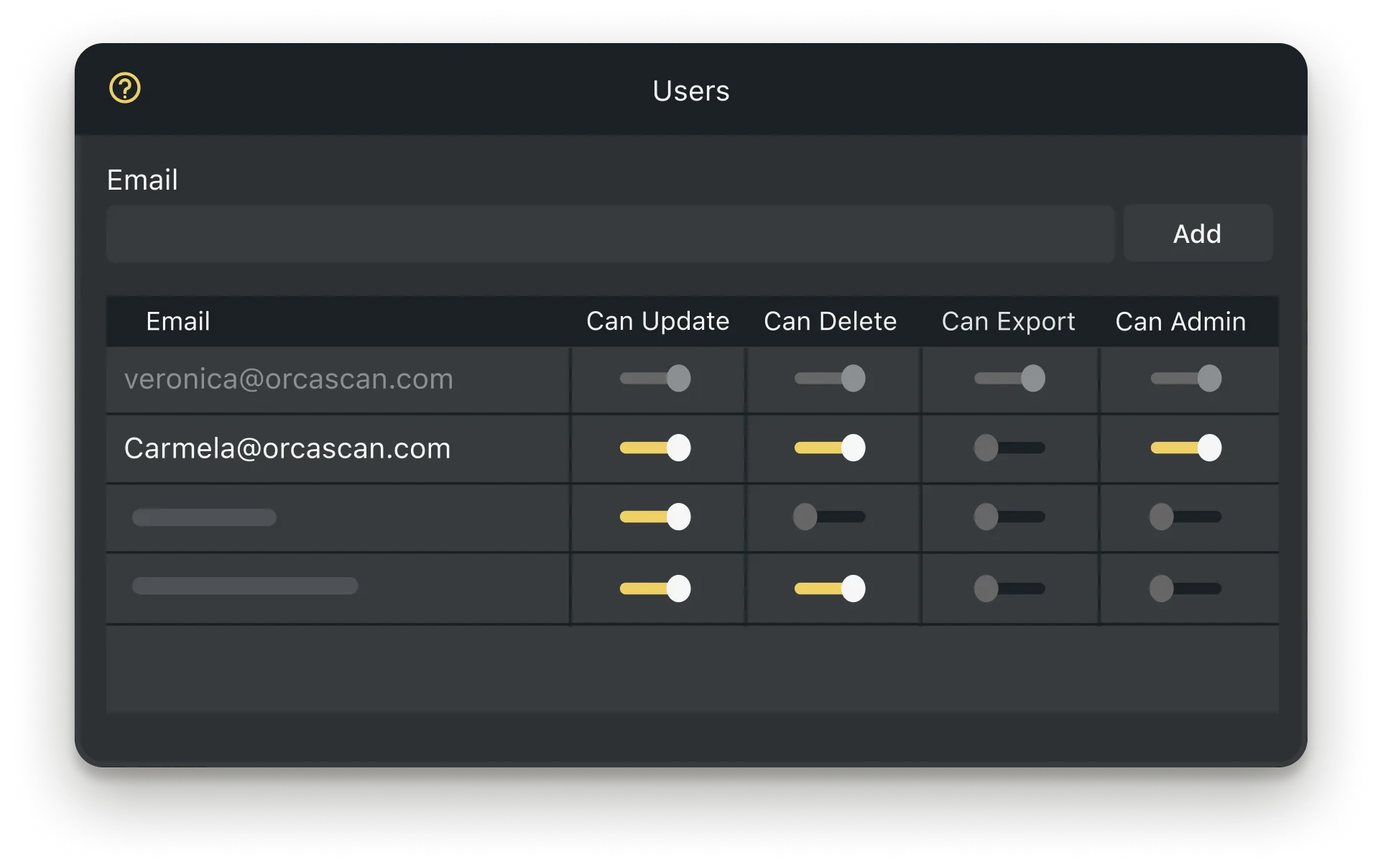
Work Online with your Team
Add users from across departments, or different hospitals sites to relevant sheets so they have access to the correct device data
-
Save Time and Effort
Damaged, lost or expired medical devices can be life-threatening. Using a digital inventory system gives you a clear, accurate view of your inventory to help save time and money
-
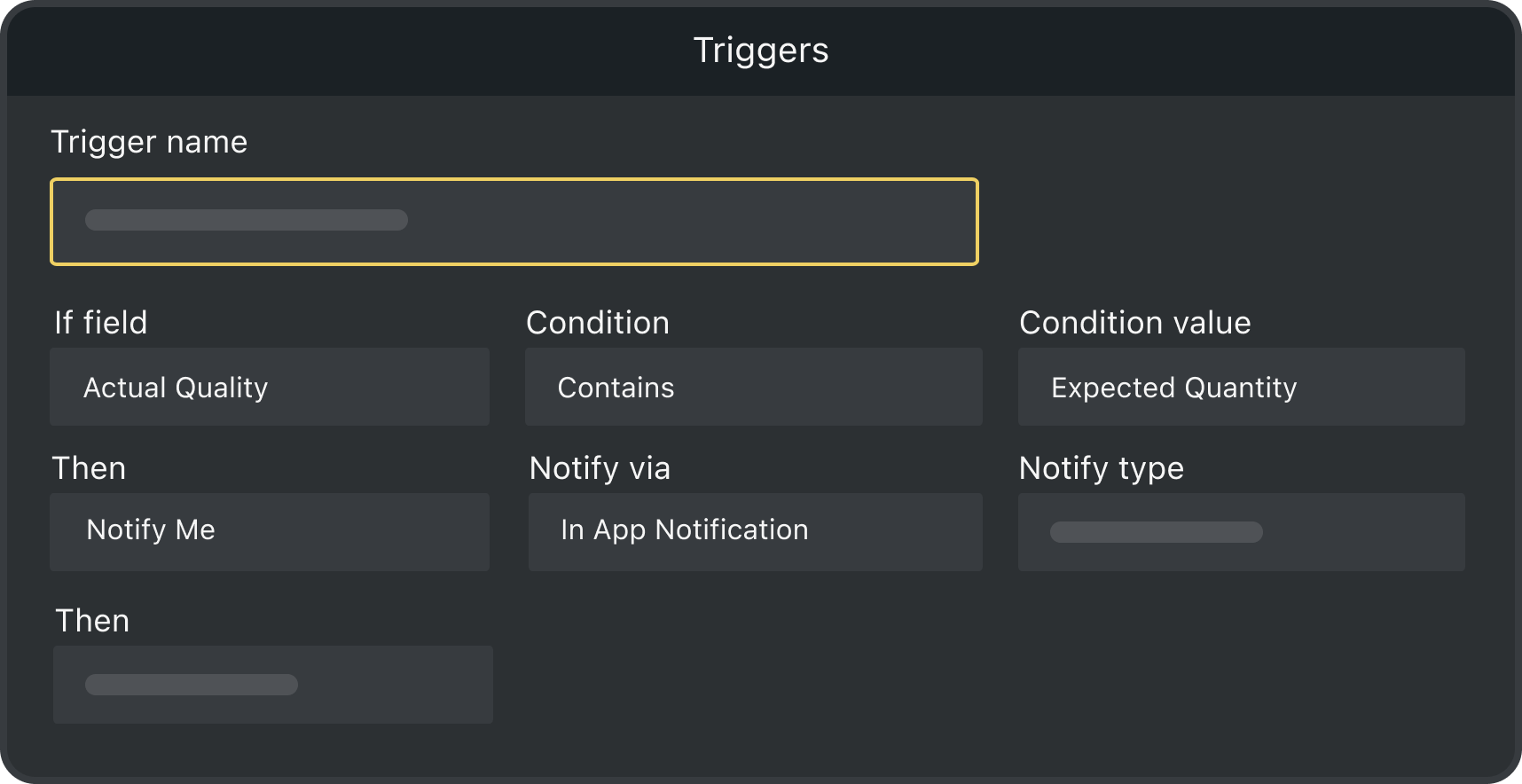
Triggers
Set up triggers to monitor stock levels, damaged products and devices, deliveries, patient updates, and more


Powerful features to improve your workflow
-
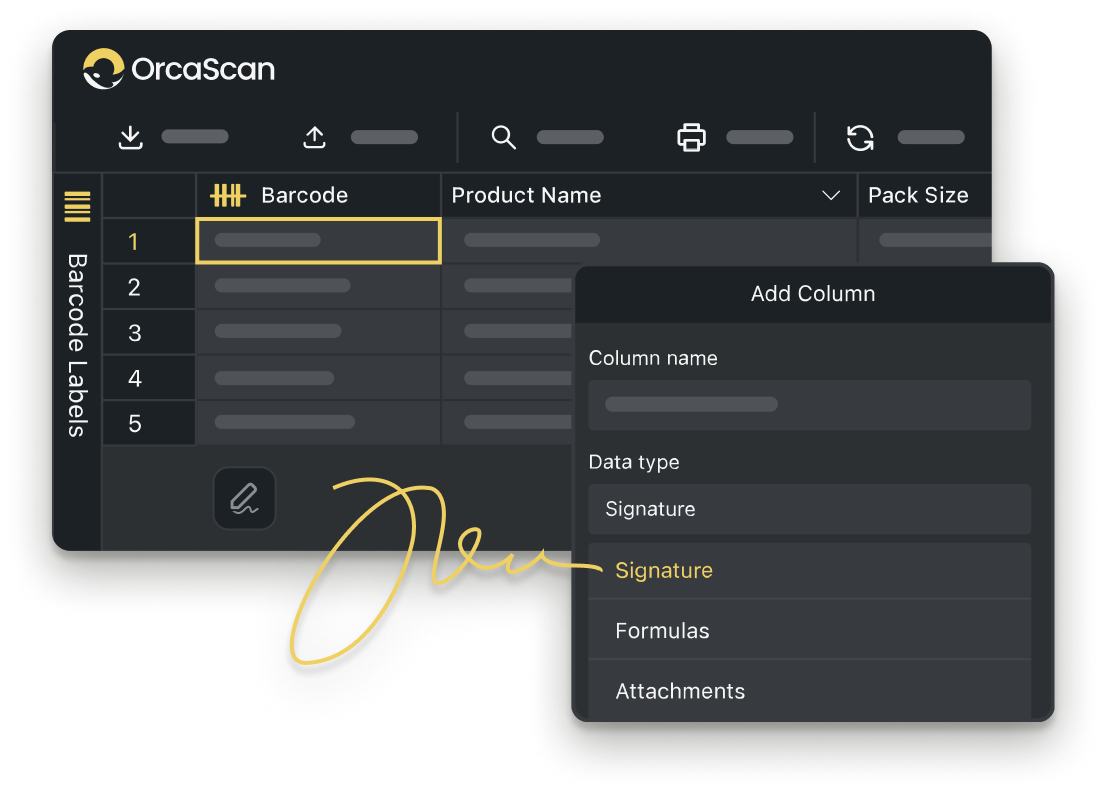
Fully Customisable
Add triggers, attachments, signatures and more by customising your Orca Scan sheet
-
Scan with Mobile
Designed to grow with you, start with smartphones and tablets and add Enterprise scanners when needed
-
Print Barcodes
If devices do not have barcodes, you can easily create design, generate and print compliant barcodes with Orca Scan
-
Export Data to Excel
Export data and reports directly to Excel to easily share with healthcare teams, other facilities, and supply chain partners
-
Add views
You can easily combine all your scanned items, from multiple sheets, into a well-organised read-only sheet with the help of views
-
Detailed History Log
Keep an accurate audit trail of who last recorded a device, edited an entry, at what location, and its condition
Medical Device Tracking FAQs
What exactly is a UDI barcode and how is it used?
UDI barcodes are made up of two parts. A device identifier with the manufacturer and device information, and a __ production identifier with the Manufacture Date, Lot Number, Serial Number and Expiration Date.
Together, they create a unique identified that lets every medical device be tracked and traced. Similar traceability needs exist in other regulated industries, including cannabis production.
Why is tracking medical devices with a UDI barcode scanner important?
Since 2016, recalls of faulty or expired medical devices have risen over 70% (Statista, 2021).
With UDI barcodes, medical professionals can instantly identify which patients received a recalled device, avoiding the invasive surgeries that were once needed to identify them.
Why can tracking UDI barcodes be difficult?
UDI barcodes can be encoded in three different formats, GS1, HIBCC and ICCBBA, each showing the same data differently. Because many systems can’t read every format, manufacturers often add multiple barcodes to products which can cause confusion to care providers who are unsure which to scan and relying on manual data entry, which only increases the risk of errors.
How can I use Orca Scan as a UDI barcode scanner?
The Orca Scan Medical Device Tracking template automatically extracts the Device Identifier, Serial Number, Manufacturer/Production Date, Lot Number and Expiration Date from a UDI barcode. If the device is registered with the US FDA, Orca Scan will also populate the manufacturer’s name and device description - reducing human error.
To get started:
- Register for an Orca Scan account or sign in to your account
- Create a new sheet using the Medical Device template
- Download the Orca Scan app
- Scan a UDI barcode and enter the quantity if needed, hit save.
To view all your medical devices, including locations, along with a full audit log, just log in to the web application.
How to enrich UDI barcodes with data from another sheet
You can automatically enrich your UDI barcode scans with details like Description, Reference Number, and Supplier Name by linking your sheet to another Orca Scan sheet containing that information:
- Prepare your master sheet
- Import a list of all device identifiers
- Rename the barcode column to
Device Identifier– this is the value that will be matched during scanning
- Allow public access to the data source
- In the master sheet, open the Integrations tab
- Enable Allow public URL access and copy the URL key
- Link your scanning sheet
- Open the child sheet you’ll use to scan UDI barcodes
- Go to the Integrations tab and paste the URL key into the Lookup URL field
- Add the parameter
?extract=$UDI:DIto the end of the URL
- Match the column names
- Make sure your child sheet has the same column names as the data source (Master Sheet)(e.g.,
Description,Reference Number,Supplier Name). - The
Device Identifierin the scanned barcode must match one in the data source.
- Make sure your child sheet has the same column names as the data source (Master Sheet)(e.g.,
Once linked, Orca Scan will check the data source every time you scan a UDI barcode. If a match is found, it will automatically pull across any matching fields into your scanning sheet.
Here's why thousands of teams count on Orca Scan
Trusted by businesses worldwide
-
If you work in the medical field and need to keep track of your medical equipment, and want to be able to customise it for your service, then Orca Scan is the solution
-
The cost savings staffing-wise is phenomenal
-
I have already recommended Orca Scan to my colleagues in other European countries
Certified and Trusted: Quality You Can Rely On
Backed by Industry-Leading Certifications
Medical Device Tracking related questions?
If you need help brainstorming how best to track your inventory, get in touch; we’d be happy to help.
















